NELLCOR BEDSIDE MONITOR SPO2 W/ REUSE PROBE *F12
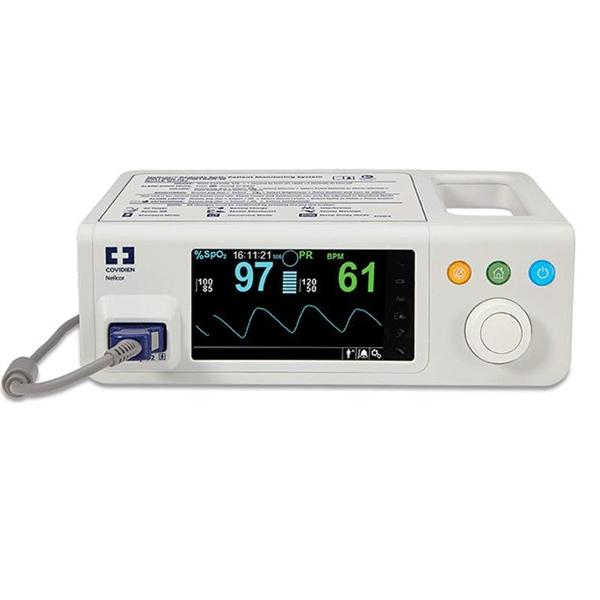
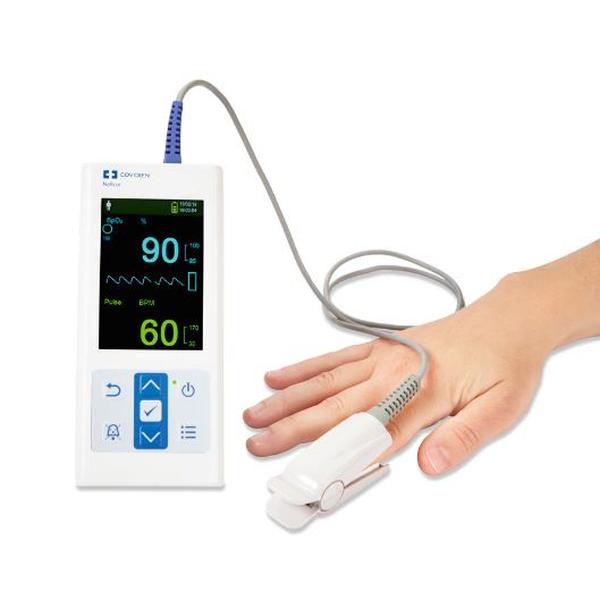
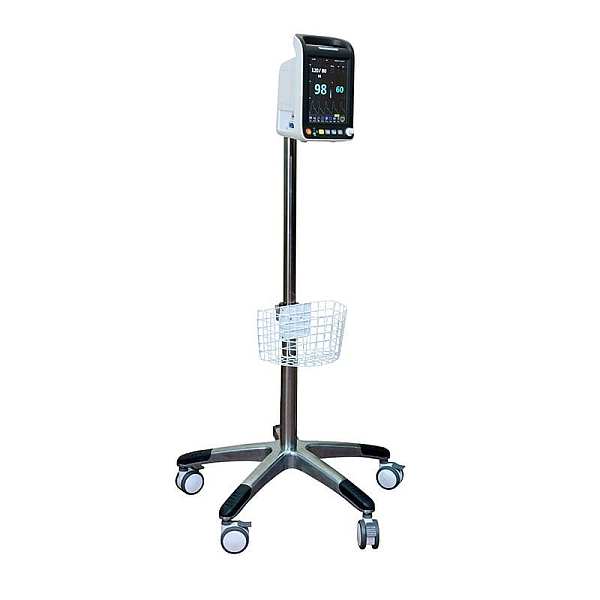
The Nellcor™ bedside SpO2 patient monitoring system incorporates the latest Nellcor™ digital signal processing technology for accurate, reliable readings even during low perfusion and other forms of signal interference, providing clinicians with access to the most critical information regarding their patients’ respiratory status.
With improved functionality, including continuous SpO2 and pulse rate monitoring, trending data and SatSeconds alarm management, clinicians have the information they need to detect respiratory complications earlier and intervene sooner.
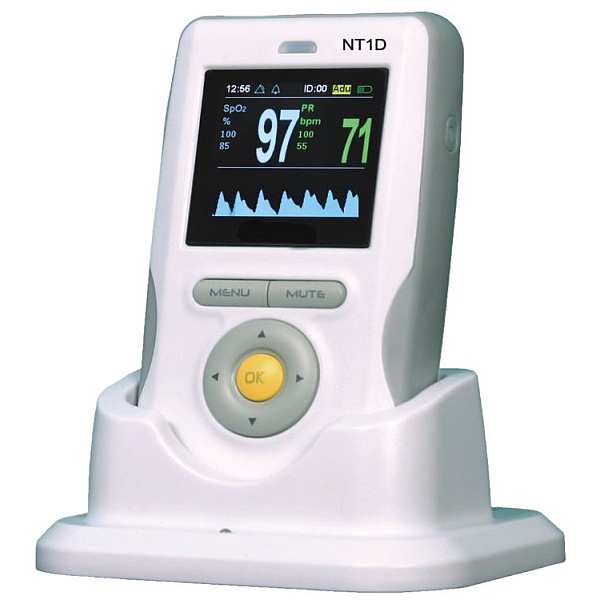
The Nellcor™ bedside SpO patient monitoring system features a compact design, improved functionality, a built-in handle and an intuitive, easy-to-read color user interface, making it simpler for clinicians to review and download critical patient data.
With its innovative features, advanced technology, and small footprint, this easy-to-use patient monitoring system helps clinicians save time and space.
FEATURES:
- Multicolor display screen with a black background for contrast
- Backup audible alarm
- Multiple-language graphical user interface to support variety of users
- 96-hour trend memory with data captured every four seconds
- Distinctive digital signal processing technology
- Simple, intuitive operation with a space-saving design
- Displays plethysmographic waveforms, pulse amplitude and current measured SpO2 and pulse rate
- A variable pitch beep tone enables clinicians to hear point-by-point changes in SpO2
- Patient trend data can be stored on a PC for archive and analysis
- An easy-to-use jog dial enables simple navigation and control of the display and monitoring system functions
- On-screen help messages assist with the use of the monitor
- Biomedical engineers and technicians can simply and effectively set institutional defaults as well as easily replace the battery, perform diagnostics to troubleshoot performance issues and generally maintain the monitor within the hospital
- COMPACT AFFORDABLE INTUITIVE
Performance
MEASUREMENT RANGE
- SpO2: 1% to 100%
- Pulse rate: 20 to 250 beats per minute (bpm)
- Pulse amplitude: 0.03% to 20%
ACCURACY
- Saturation: (% SpO2 ± 1 SD)
- Adult: 70% to 100% ± 2 digits
- Neonate: 70% to 100% ± 3 digits
- Low perfusion: 70% to 100% ± 2 digits
- Pulse rate: 20 to 250 bpm ± 3 digits
- Low perfusion: 20 to 250 bpm ± 3 digits
Output
- Trend data download
- Nurse call capability
Display/Indicators
- Pulse: Amplitude indicator (eight segments)
- Visual indicators: Pulse search, audible alarms silenced or off, interference indicator, battery charging, SatSeconds alarm management clock, pleth
Alarms
- Audible and visual alarms for high/low saturation and pulse rate
- SatSeconds alarm management settings: 10, 25, 50 and 100, or off
- Audible and visual warning indicators for low battery and sensor off
- Audible and visual sensor disconnect alarms
Optional Accessories
- Mounting adapter
- Interface cables
- 10-hour battery
Electrical
INSTRUMENT
- Power requirements: 100 to 240 VAC, 50/60Hz, 45 VA
- Fuse rating: Fast-acting 2A 32VAC/DC, fast-acting 500mA 32VAC/50DC
BATTERY
- Type: Lithium ion
- Battery capacity: Minimum of five hours using new, fully charged battery with no alarms
Environmental
OPERATING TEMPERATURE
- Instrument: 5°C to 40°C (41°F to 104°F)
- Transport/storage temperature (in shipping carton): -20°C to 60°C (-4°F to 140°F)
OPERATING HUMIDITY
- 15% to 93% noncondensing
OPERATING ALTITUDE
- -170 m to 4877 m (-557 ft to 16,000 ft)
Physical Characteristics
WEIGHT
- 1.6 kg (3.5 lbs)
SIZE
- 82 H x 255 W x 165 D (mm)
- (3.23 H x 10.04 W x 6.50 D (in))
Equipment Compliance
STANDARDS COMPLIANCE
- EN ISO 9919: 2009, EN ISO 80601-2-61:2011
- EN IEC 60601-1: 2005
- EN IEC 60601-1-2: 2nd edition
- EN IEC 60601-1:1998 + A1:1991 + A2:1995
- EN 60601-1:1990 + A11:1993 + A12:1993 + A13:1996
- CAN/CSA C22.2 No. 601.1 M90
- UL 60601-1: 1st edition
Equipment Classifications
- Type of protection against electric shock: Class 1 (internally powered)
- Degree of protection against electric shock: Type BF - applied part
- Mode of operation: Continuous
- Electromagnetic compatibility: IEC 60601-1-2:2007
- Liquid ingress: IPX2
- Degree of safety: Not suitable for use in the presence of flammable anesthetics
Related Products